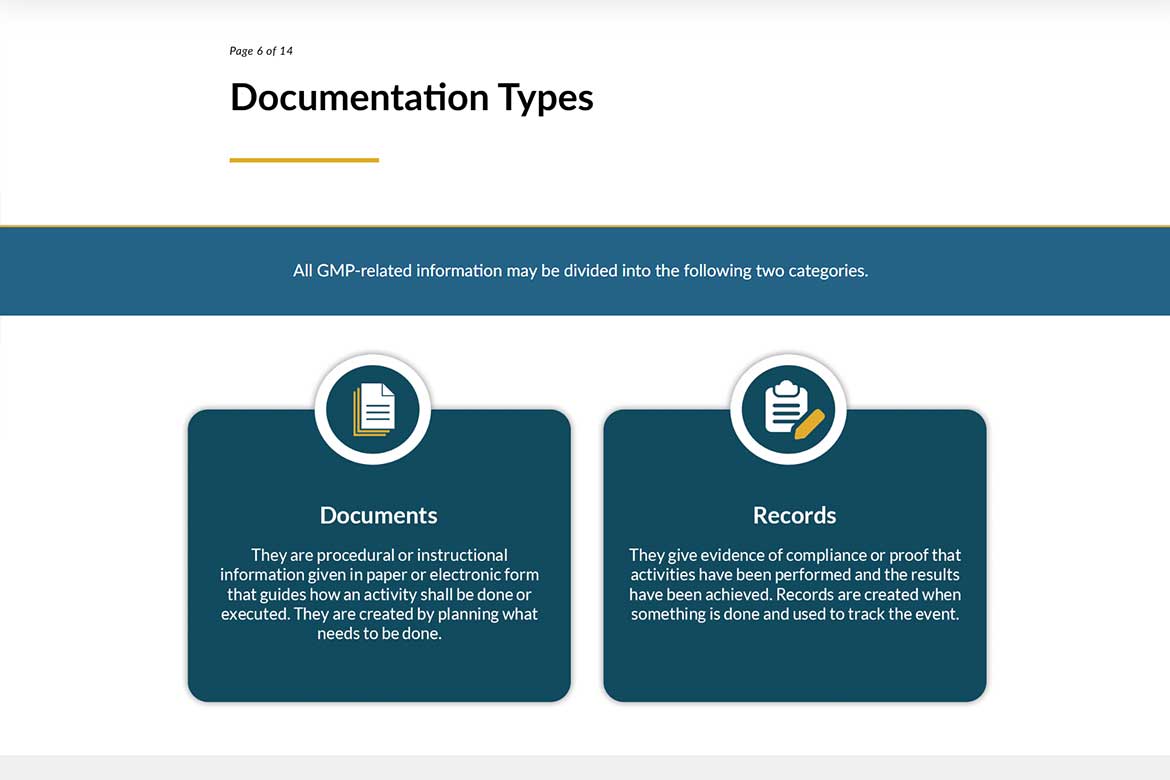
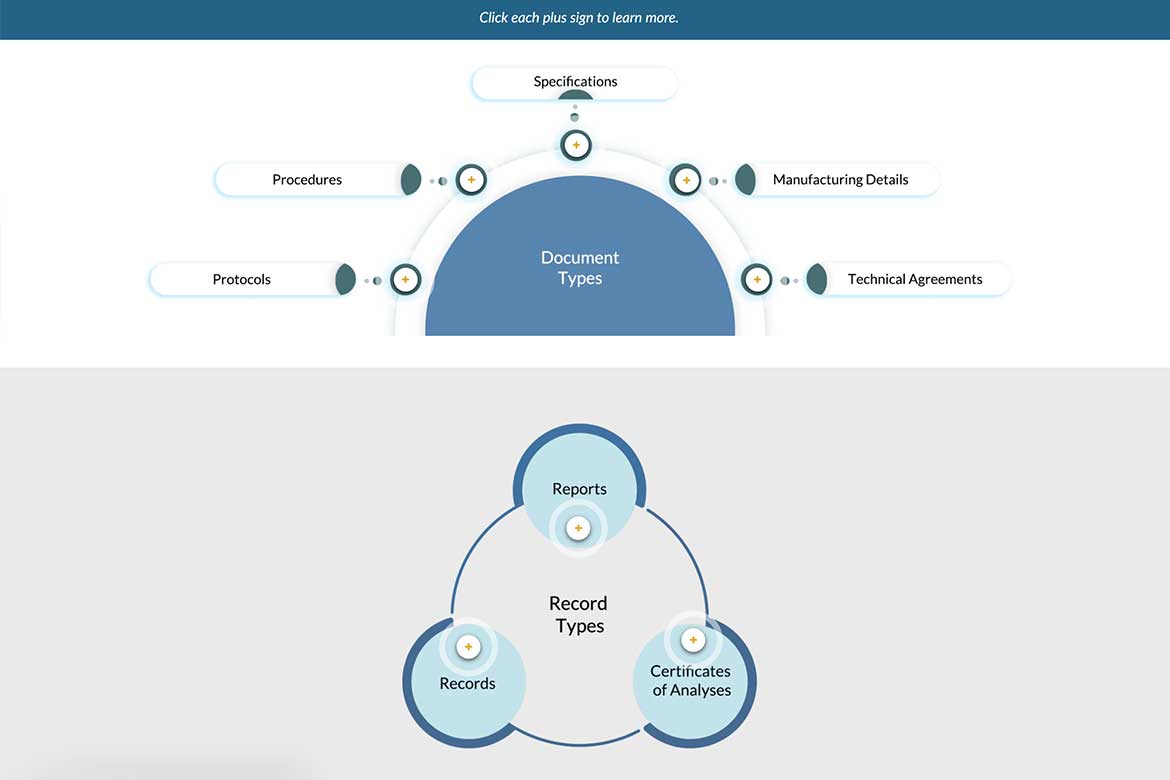
This module offers a clear introduction to GMP documentation—explaining the difference between documents and records, key documents used in Production and Quality Control, and the purpose of a Site Master File.
This Articulate Storyline module, crafted with strong instructional design, introduces learners to the fundamentals of GMP documentation. It clearly explains the difference between documents and records, outlines essential documentation for Production and Quality Control, and demystifies the purpose of the Site Master File. Learners also gain access to a practical checklist to ensure all required information is captured accurately and compliantly.